Products
Latest News
Photoinitiators
🔹 Introduction

A photoinitiator is a molecule that creates reactive species (free radicals, cations or anions) when exposed to radiation (UV or visible). They initiate the polymerization process by adding themselves to the monomer or oligomer.
🔹 Classification of photoinitiators
Photoinitiators can be divided into two classes radical photoinitiators and cationic photoinitiators.
Radical photoinitiators can react differently under UV-irradiation. Depending on their reaction they are classified as Norrish Type I or Norrish Type II Photoinitiators.
- Norrish Type I photoinitiators(cleavage)
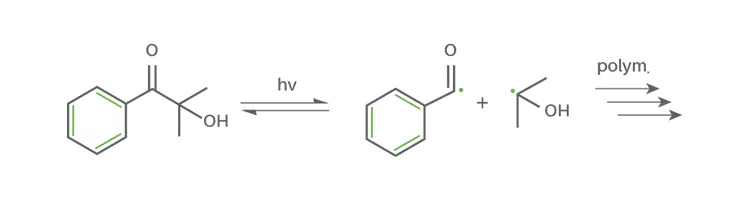
Norrish Type I photoinitiators are characterized by a cleavage reaction into two radical fragments of the original photoinitiator. The irradiation with UV-light leads to a homolytic bondage cleavage and generation of two highly reactive radical species. These radicals then initiate the polymerization. The Type I photoinitiator is irreversibly incorporated into the polymer matrix.
- Norrish Type II photoinitiators(abstraction)
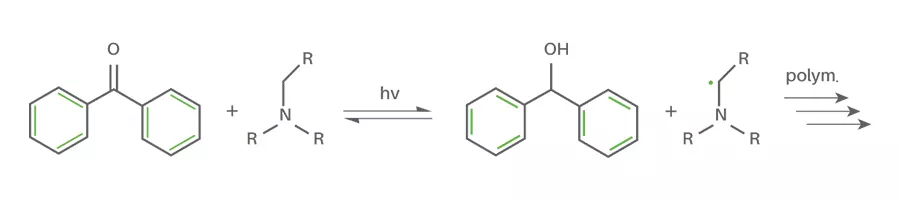
Norrish Type II photoinitiators, when irradiated by UV-light, need a hydrogen donor to react. Most commonly these hydrogen donors are amines (amine synergists). By UV-irradiation the Type II photoinitiator abstracts a hydrogen atom from the employed synergist forming two radicals. These radicals like the Type I photoinitiators can then initiate the polymerization reaction. Type II photoinitiators normally are not incorporated during the reaction but the synergist is.
- Cationic photoinitiators
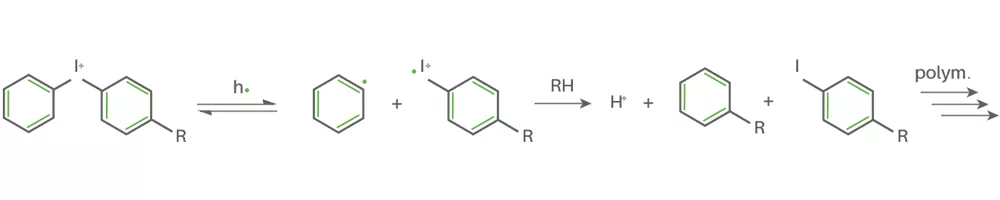
Cationic Photoinitiators react very differently to the previous mentioned Type I/II Photoinitiators. Mainly iodonium and sulfonium salts are employed as cationic photoinitiators. When irradiating these salts with UV-light they undergo, like Type I photoinitiators, homolytic bondage cleavage. The formed radicals react with a proton donor to a Brønsted or Lewis acid. The generated acid then initiates the polymerization.
🔹 Applications and Advantages
Photoinitiators are used extensively together in combination with cross-linkable monomers and oligomers in ultra-violet-curable inks and coatings, adhesives and many other products.
Ultra-violet curing technology represents an environmentally-friendly and cost-effective alternative to other technologies such as solvent based formulated systems, as there is no water to remove at the end of the printing or coating process, and no solvents to capture or incinerate.
UV curable coatings can offer improved performance and visual properties. One of the main advantages from an environmental perspective is the absence of solvents. Further there are energy savings because of faster start-ups and shut-downs – UV lamps turn on and off almost instantaneously. Other advantages of UV-curing include the fact are that they are much faster than thermal processes. UV coatings cure in a matter of seconds, rather than minutes or hours. Productivity is improved as UV coatings cure in a matter of seconds.






