【Synergistic Enhancement of Dual Mechanisms】The Photochemical Dance between Thioxanthones and Photoinitiator 907: Solving the Curing Problem of Colored Systems
Abstract:
In the light curing technology, due to the competitive light absorption effect of pigments, colored systems often face challenges such as low curing efficiency and incomplete reaction. This article deeply analyzes the synergistic effect of the dual mechanisms between thioxanthone-based photoinitiators and α-aminoketone-based photoinitiator 907, providing an efficient solution for colored UV curing.
1. Product Characteristics of Photoinitiator 907
★ Chemical Name:
2-Methyl-1-(4-methylthiophenyl)-2-morpholinopropan-1-one
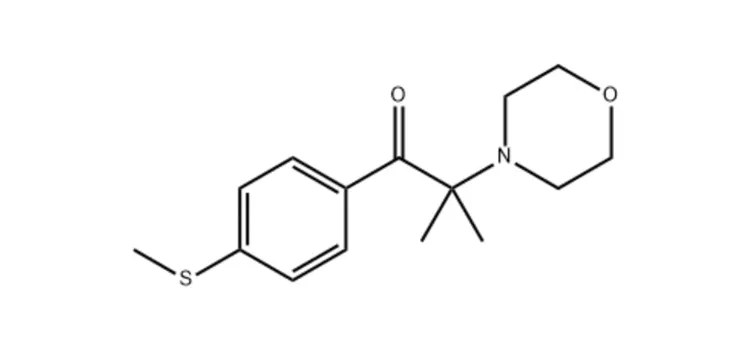
★ Appearance: White powder
★ Melting Point: 70-75 °C
★ Absorption Peaks: 232 nm, 307 nm
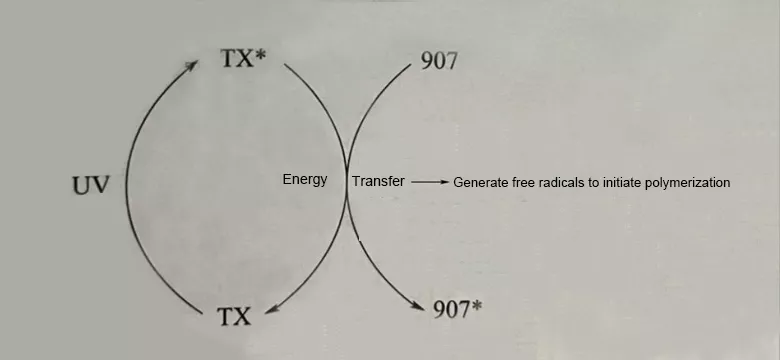
2. Principle of Synergistic Initiation
When 907 undergoes photolysis, it generates p-(methylthio)benzoyl radicals and morpholinyl isopropyl radicals, both of which can initiate polymerization. In a colored system, due to the light absorption of pigments, the efficiency of the photoinitiator is greatly reduced. It is recommended to use it synergistically with thioxanthone-based photoinitiator ITX. When used synergistically, the absorption wavelength can reach 360 nm - 405 nm, and it is compatible with LED UV light sources.
The synergistic effect between α-aminoketone-based photoinitiator 907 and thioxanthone-based initiators realizes the efficient curing of colored systems through a dual photochemical mechanism:
Mechanism 1: Energy Transfer Sensitization (Triplet State Path)
★ Thioxanthones preferentially absorb photons in the long-wavelength ultraviolet region (380-420 nm), breaking through the light shielding effect of pigments.
★ After being excited to the triplet state, it undergoes Förster resonance energy transfer (FRET) with photoinitiator 907, prompting 907 to transition to the excited state.
★ Thioxanthones can recycle and participate in the reaction after returning to the ground state.
Mechanism 2: Electron Transfer Activation (Amine Synergy Path)
★ The morpholinyl group (tertiary amine structure) in the photoinitiator 907 molecule forms an exciplex with thioxanthones.
★ Through intermolecular electron transfer (PET), α-aminoalkyl radicals are generated, initiating chain polymerization.
3. Advantages of Synergistic Use
When the two are used synergistically, the quantum yield can reach 0.7-0.9, which is 2 to 3 times higher than that of a single-component photoinitiator. Therefore, the synergistic use of thioxanthone-based photoinitiator (ITX) and α-aminoketone-based photoinitiator (907) is particularly suitable for colored photocuring systems and black photocuring systems.
However, the photolysis products of photoinitiator 907 are sulfur-containing compounds, which have a certain odor, and there are also certain problems with yellowing resistance, so it is not recommended to use it in varnishes and white paints.
Technical data reference:
Journal of Photochemistry and Photobiology A: Chemistry (2018)
Progress in Organic Coatings (2020)
For product inquiries or technical consultations, please feel free to contact us.
We are looking forward to cooperating with you!





