Thiol-ene Photocuring System: Mechanism, Characteristics and Applications
Abstract
Thiol-ene photopolymerization is a UV-initiated step-growth polymerization reaction that enables the synthesis of polysulfide polymer networks with diverse properties. Endowed with its unique reaction mechanism, this system exhibits remarkable advantages including low oxygen inhibition, low shrinkage rate, high conversion rate and deep curing. It holds substantial application potential in fields such as electronics and adhesives, yet it also faces challenges like thermal sensitivity and poor storage stability.
Thiol-ene systems composed of thiols and a series of different unsaturated compounds can synthesize polysulfides through stepwise photopolymerization. Polysulfides are a distinct class of polymers with diverse properties and application fields.
I. Core Reaction Mechanism
This polymerization process involves the stepwise addition of thiol to alkenyl groups initiated by a UV-controlled free radical source. Initially, a sulfur-centered radical is formed. Thiol, as an optimal hydrogen donor, can react with both the free radicals generated by Type I photoinitiators and the excited triplet states of Type I photoinitiators.
The reaction between sulfur radicals and unsaturated bonds produces carbon-centered radicals. Such alkyl radicals can abstract a hydrogen from a second thiol molecule, forming another sulfur radical and sustaining the polymerization process continuously.
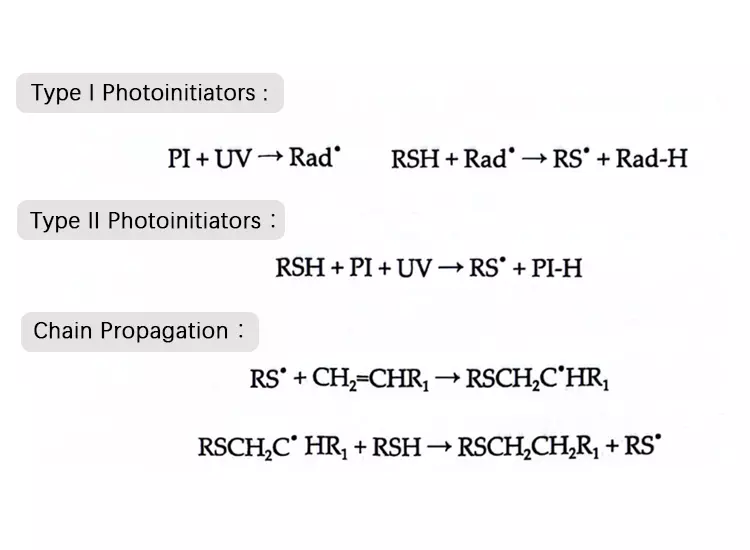
✿ 1. Initiation:
Ultraviolet light activates the photoinitiator (e.g., Type I initiator), generating free radicals or excited triplet states.
✿ 2 . Hydrogen Transfer:
The initiator radical abstracts a hydrogen atom from a thiol (R-SH) molecule, forming a sulfur-centered radical (thiyl radical).
✿ 3. Addition Reaction:
The thiyl radical attacks the unsaturated double bond (C=C) of the alkene, producing a carbon-centered radical.
✿ 4. Chain Transfer:
This carbon-centered radical abstracts a hydrogen atom from another thiol molecule, generating a new thiyl radical and a consumed alkene molecule.
✿ 5. Chain Growth:
The newly formed thiyl radical continues to react with alkene double bonds, cycling through steps 3 and 4 to sustain the polymerization reaction, ultimately forming a crosslinked polymer network.
II. System Characteristics
Advantages:
✿ 1. Low Oxygen Inhibition:
Peroxide radicals generated by oxygen can be effectively reduced by thiols, regenerating active thiyl radicals, so the reaction is not inhibited by oxygen. It can also act as an oxygen scavenger.
✿ 2. Low Shrinkage Rate:
The volume shrinkage rate from liquid monomers to solid polymers is extremely low (only 3%-5%), which helps to achieve good adhesion and reduce internal stress.
✿ 3. High Conversion Rate and Deep Curing:
Fast curing rate, high monomer conversion rate, capable of curing thick cross-sections (up to 1 cm), and the products have high optical transparency.
✿ 4. Tunable Performance:
By selecting thiols with different functionalities (e.g., trimethylolpropane tris(3-mercaptopropionate)) and alkene monomers, various materials ranging from flexible elastomers to rigid plastics can be designed.
✿ 5. Wide Monomer Selection & High Reactivity:
A variety of alkenyl monomers (e.g., vinyl ethers, norbornenes) can participate in the reaction. Among them, norbornene has extremely high reactivity, requiring only one-tenth of the curing energy of acrylates.
✿ 6. Flexible Photoinitiator Selection:
Common Type I photoinitiators (e.g.,photoinitiator 1173, photoinitiator 184) or Type II photoinitiators can be used; even under high-intensity short-wave UV light, thiols can directly cleave to generate free radicals, enabling initiator-free curing.
Disadvantages:
✿ 1. Poor Storage Stability (Dark Reaction):
The formulation is thermally sensitive and may undergo slow thermally initiated reactions during storage, leading to pre-gelation and a limited shelf life.
✿ 2. Raw Material Odor:
Thiol raw materials usually have an unpleasant odor (though the odor is mild after curing).
III. Application Fields
Although not yet widely popularized, the thiol-ene system has been applied in the following fields:
✿ Electronic Industry:
Used as conformal coatings.
✿ Adhesives and Sealants:
Employed in the preparation of adhesive and sealing materials.
✿ Printing Field:
Applicable to special printing processes.
If you encounter surface drying issues or sensitivity problems, you can try our trifunctional thiol YS-623 or tetrafunctional thiol YS-624.
We welcome inquiries at any time and look forward to achieving win-win cooperation with you!
Previous: Process Principle and Formula Design of UV Vacuum Plating Coatings
Next: No More





